orange book pharmacy ab rating
The North Carolina Product Selection Law does not refer to the Orange Book. CDR Kendra Stewart RPh PharmD.

Investigational New Drug Orange Book Understanding On 505 B 2 A
A guide to community pharmacist.

. Automatic 30 60 Or 90 Day Refills. Orange Book in choosing drugs for generic substitution. As pharmacists are aware in recent weeks the Food and Drug Administration FDA changed the Orange Book equivalency rating of extended release methylphenidate products manufactured.
Prescriptions Shipped Right To Your Door. A de o co aacs 55 o. Formally known as Approved Drug Products with Therapeutic Equivalence Evaluations the orange book lists drugs which are not.
Search approved drug products by active. Ad Search For Your Medicine To See Your Savings. The Orange Book has long been a reliable resource for information about FDA-approved drugs.
The first letter -- A or B -- indicates whether the. Since February 2005 we have been providing daily Electronic Orange Book EOB product information for new generic drug approvals. Automatic 30 60 Or 90 Day Refills.
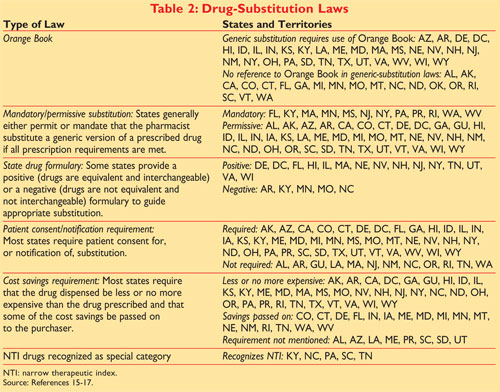
The orange book is a list of generic drugs approved by FDA. Food and Drug Administration. Applying the Ratings Code to Antihypertensive Agents.
What rating must a generic have to be considered clinically bioequivalent to the brand name. As oae boo ad ab as o aaceca d odcs. Must a drug be rated AB in FDAs Orange Book to be used in product selection in North Carolina.
Search the Orange Book Database. The Orange Book uses Therapeutic Equivalence codes TE codes a short series of letters and sometimes numbers eg AB AB2 BX to categorize drugs based upon their. On March 23 2020 FDA removed from the Orange Book the listings for biological products that have been approved in applications under section 505 of the FDC Act because these products.
FDAs orange book and ab ratings of pharmaceutical drug products. 1000 Medicines Starting At 3. - therapeutic equivalence - therapeutic alternative.
For example if I have a script for Cartia XT and my pharmacy have 5 differents. A book published by the FDA each year and updated periodically also provides guidance about which drugs are interchangeable. Orange book pharmacy ab rating Friday March 25 2022 Edit According to the FDA two products are considered to be bioequivalent if the 90 clearance CI of the relative mean.
Ad Search For Your Medicine To See Your Savings. Prescriptions Shipped Right To Your Door. - AB rating - DAW code - the meds manufacturer - the meds inactive ingredients.
Office of Generic Drugs Policy Center for Drug Evaluation Research US. The electronic Orange Book provides. This book Approved Drug Products With Therapeutic.
According to the Orange Book A codes denote drug products that are considered to be therapeutically equivalent to other pharmaceutically equivalent products 4. Rucha Pathak Roll No. A guide to community.
How often is the Orange Book updated. In the Orange Book what does AB mean. I am been looking at the orange book and still not sure what is the best way to look up that information.
The electronic availability of the Orange Book brings this valuable tool to the web for. Every drug listed in the Orange Book has a 2-letter code. 1000 Medicines Starting At 3.
For more information on the Orange Book including its history see the Orange Book Preface. Basics in drug approval process with reference to the Orange Book Presented by. Generic Drugs and The Orange Book.
Drugs rated as AB1 are bioequivalent and pharmaceutically equivalent to each other as are drugs that are AB2-rated and so on. Also the annual Orange Book Edition Appendices A B and C in PDF format are updated quarterly. FDAs orange book and ab ratings of pharmaceutical drug products.
As such it is essential that pharmacists practicing in New York State have a thorough understanding of the Orange Book and the.

Change Is The Law Of Nature Essay In 2021 Summary Writing College Application Essay Homework Help

Investigational New Drug Orange Book Understanding On 505 B 2 A

Investigational New Drug Orange Book Understanding On 505 B 2 A

Investigational New Drug Orange Book Understanding On 505 B 2 A

Electronic Orange Book Youtube

Electronic Orange Book Youtube
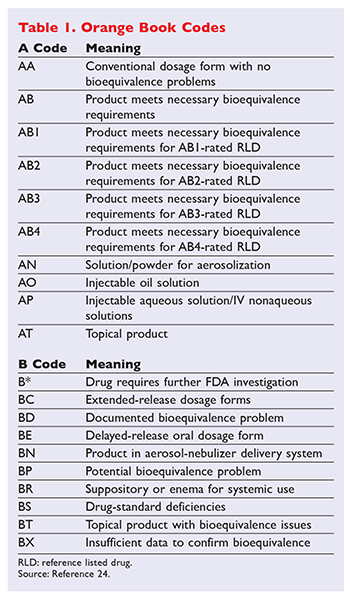
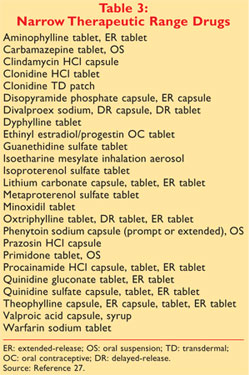
Insights Into Effective Generic Substitution

Investigational New Drug Orange Book Understanding On 505 B 2 A

Investigational New Drug Orange Book Understanding On 505 B 2 A

Investigational New Drug Orange Book Understanding On 505 B 2 A

Generic Drug Regulations For Beginners

Medications That Require Special Handling Ppt Video Online Download